Background: Hemophagocytic lymphohistiocytosis (HLH) is a rare, life-threatening syndrome caused by activation of T cells that results in extreme inflammation. HLH is commonly associated with underlying conditions such as infection, autoimmune disease, or malignancy. HLH management includes treatment directed at the specific trigger, suppression of maladaptive cytokines, and inhibition of T cell and macrophage proliferation. Granulocyte colony-stimulating factor (GCSF) is a myeloid growth factor that stimulates the production, maturation, and activation of neutrophils. GCSF may theoretically promote production of inflammatory cytokines and enhance hemophagocytosis, thereby intensifying HLH. By contrast, GCSF could play a vital role in the treatment of granulocytopenia caused by HLH and thus limit the development of infections. There is a paucity of data examining the role of GCSF in patients with HLH. Current literature is limited to case reports of HLH exacerbated by GCSF administration. Practice patterns of GCSF use in HLH are highly variable, both within and between institutions.
Aim: To compare the clinical course and overall survival of patients with HLH who received GCSF treatment and those who did not.
Methods: We performed a retrospective, single-center cohort study of patients admitted with a diagnosis code of HLH from July 1997 through February 2023. Patients at least 18 years old with active HLH were included. Each case was reviewed to confirm the diagnosis of HLH based on available diagnostic evaluation and the final discharge diagnoses as documented by the primary treating team. In cases indeterminate for HLH, a second independent reviewer performed additional evaluation to reach consensus. The primary outcome was overall survival as determined by the Kaplan Meier method with statistical comparison using the log-rank test. Other examined outcomes included: incidence of infection, length of hospital stay (LOS), incidence of intensive care unit (ICU) admissions, length of ICU stay, incidence of 30-day re-admissions due to HLH, and time to first change in HLH-directed therapy.
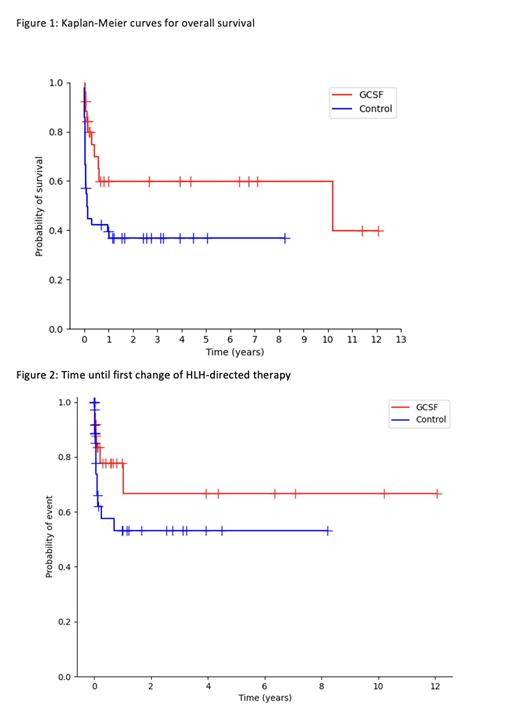
Results: Database analysis revealed 130 patients with a diagnosis code of HLH, of which 68 patients met inclusion criteria. The study cohort comprised 26 patients that received GCSF treatment and 42 patients that did not. The median follow-up time was 3.1 months (interquartile range [IQR] 0.6-29.4). The most common HLH triggers were infection (30.8% GCSF group vs 45.2% control, p=0.31) and malignancy (65.5% GCSF group vs 19% control, p<0.001). A median of 10 doses (IQR 6-16) of GCSF were administered per patient in the GCSF group, and the median time between diagnosis and the first administered dose was 6.5 days (IQR 2.25-18.75). Median overall survival in the GCSF group was 122.6 months compared to 1.5 months in the control group (p=0.02, Figure 1). There was a higher but statistically insignificant difference in infection rate in the GCSF arm (92.3%) compared to the control arm (71.4%, p=0.06). The median LOS was higher in the GCSF group (38 days vs 15 days, p<0.001). Rates of ICU admission (53.4% vs 62%, p=0.69), intubation and mechanical ventilation (34.6% vs 33.3%, p=1.0), and initiation of renal replacement therapy (23.1% vs 16.7%, p=0.74) were similar across the GCSF and control groups, respectively. Two patients in each group were re-admitted for HLH within 30 days of initial discharge. The median time to change in HLH-directed therapy was not reached in either group, and there was no significant difference in incidence of therapy change between groups at 1, 3, or 6 months (Figure 2).
Conclusions: In this single center, retrospective cohort study of patients with HLH, treatment with GCSF was not associated with inferior overall survival. While over-representation of a malignancy-associated trigger in the GCSF group may confound interpretation of this result, it nevertheless reflects an important clinical finding that contradicts previously published case report-level evidence. This study highlights the need for further prospective investigation of GCSF administration in HLH.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Filgrastim or a biosimilar was used in patients with neutropenia in the setting of hemophagocytic lymphohistiocytosis.